Our Pipeline
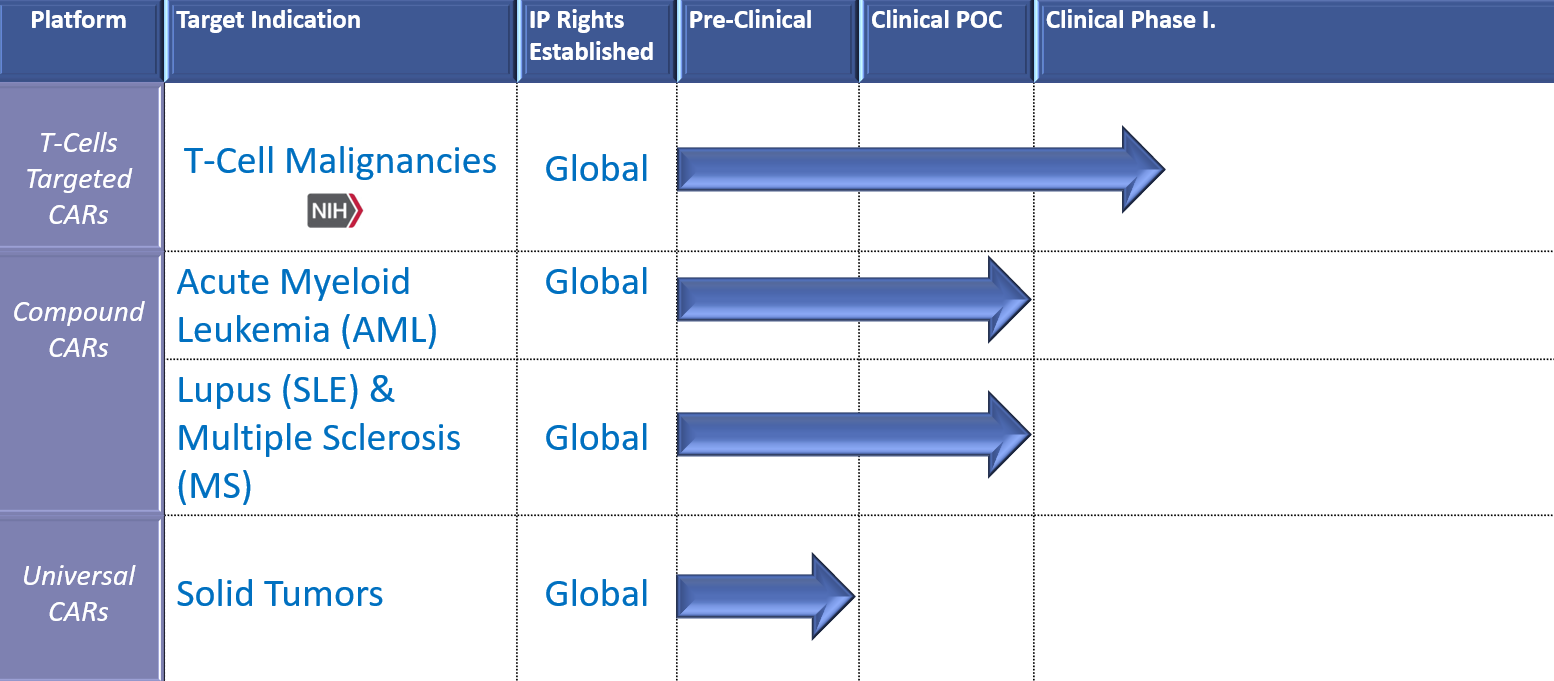
iCell Gene Therapeutics researches and develops Chimeric Antigen Receptor (CAR) engineered cell therapies for terminally ill patients where no treatment option exists and where dramatic increase in quality and duration of life is predicted.
The US Food and Drug Administration has granted Orphan Drug Designation for seven of our CARs: in Multiple Myeloma, Peripheral T-cell Lymphoma, Acute Myeloid Leukemia, B-Acute Lymphocytic Leukemia, and T-cell Acute Lymphocytic Leukemia.
Our operations are focused on creative and efficient paths to bring novel, cost effective therapies to patients. First-in-class clinical studies are ongoing in the US and in China:
Treating: Systemic Lupus Erythematosus (Lupus) & Multiple Sclerosis Neuromyelitis Optica Spectrum Disease
Our novel compound CAR (cCAR) technology treats Systemic Lupus Erythematosus (Lupus) and Multiple Sclerosis Neuromyelitis Optica Spectrum by addressing the root cause of illness. Our cCAR treatment destroys disease causing memory B cells and long-lived plasma cells thus depleting autoantibodies. This highly efficacious approach targets surface antigens CD19 and BCMA with exceptional precision and accuracy maximizing patient safety. Clinical outcomes have been encouraging with our treated patients disease free for up to three-years.
Clinical reference: Treatment of Systemic Lupus Erythematosus using BCMA-CD19 Compound CAR - PMC (nih.gov)
Treating: Acute Myeloid Leukemia (AML)
Our novel compound CAR (cCAR) technology treats acute myeloid leukemia (AML) by targeting malignant cells with great accuracy and precision. Our cCAR treatment destroys disease causing leukemia stem cells (LSCs) and bulky tumor mass. This highly efficacious approach targets surface antigens CD33 and CLL-1 with exceptional precision and accuracy maximizing patient safety.
Clinical References:
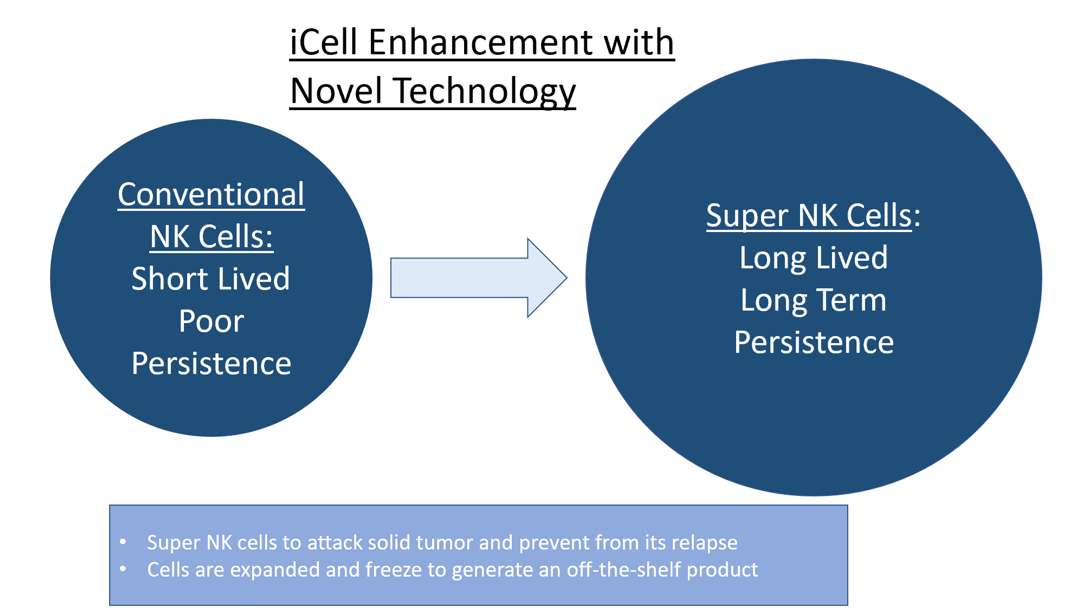
Super NK Cells Treat Soft Tumors
Our novel Super NK cell technology treats solid tumors by targeting malignant cells with great accuracy and precision. These highly effective Super NK cells minimize disease and prevent relapse which could be utilized in an off-the-shelf capacity maximizing access to patients in need.
Seven Orphan Drug Designations
Our seven novel platforms listed qualify for orphan drug designations which are granted to treat serious or life-threatening conditions that affect fewer than 200,000 people in the United States. Such drugs receive this classification if they show significant promise in treatment of orphan diseases with unmet clinical needs.
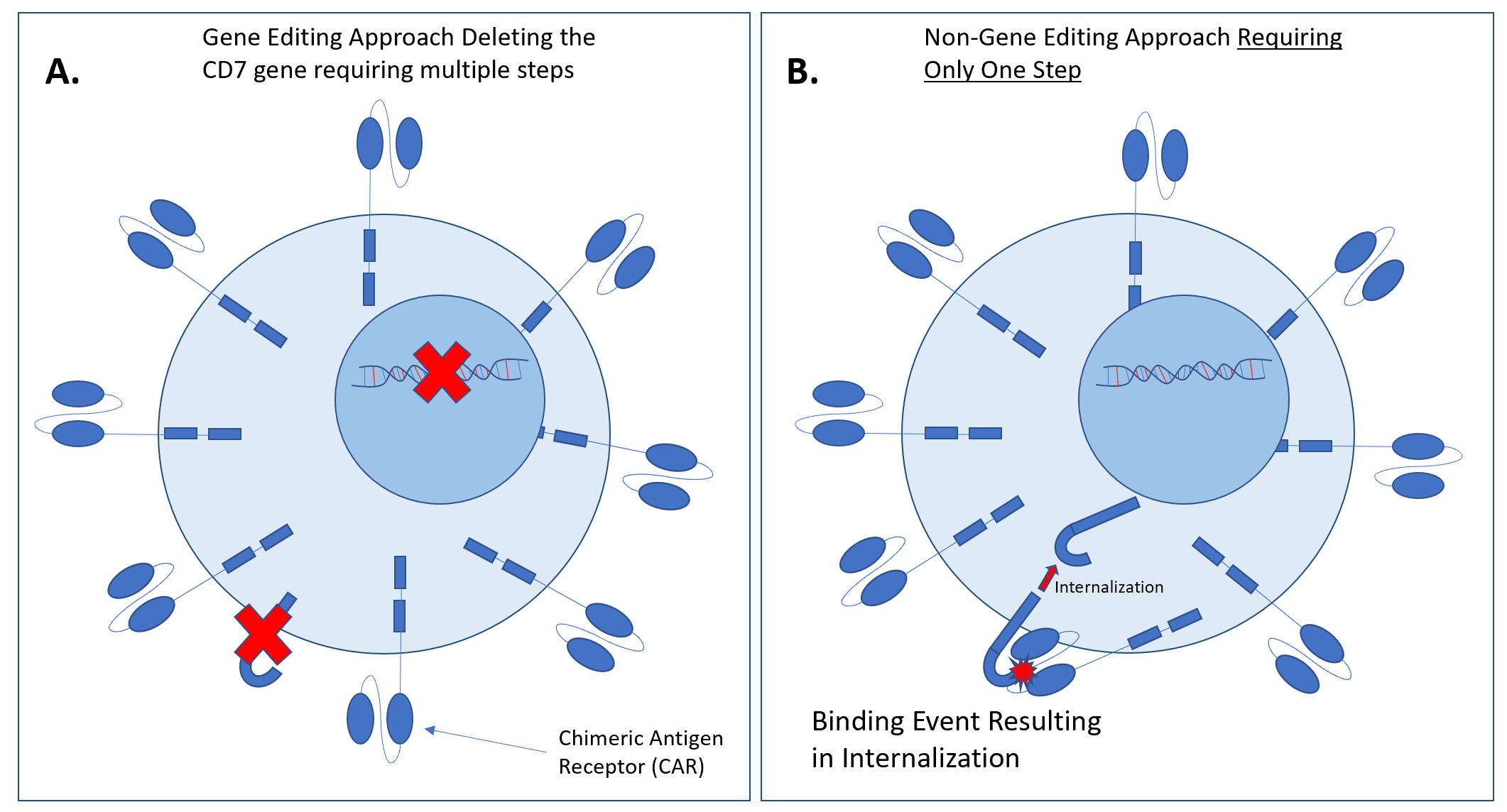
Non-Gene Editing CAR T-cell for Treating T-cell malignancies
Our novel CAR T-cell constructs target CD4, CD5, and CD7 surface antigens with great accuracy and precision respectively. Our non-gene editing approach requires only one step, and results in internalization following binding. This highly efficacious approach exhibits potent cytotoxicity depleting malignant blasts while maximizing patient safety and maintaining complete remission.
Clinical References: